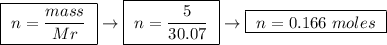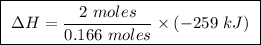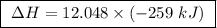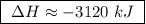Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
At constant pressure, the combustion of 5.00 g of c2h6(g) releases 259 kj of heat. what is δh for th...
Questions



Mathematics, 08.09.2021 20:30

History, 08.09.2021 20:30

Mathematics, 08.09.2021 20:30

Business, 08.09.2021 20:30


Mathematics, 08.09.2021 20:30


Biology, 08.09.2021 20:30

Biology, 08.09.2021 20:30

Social Studies, 08.09.2021 20:30

Biology, 08.09.2021 20:30





Mathematics, 08.09.2021 20:30


Mathematics, 08.09.2021 20:30

 .
.