

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
The equilibrium constant is given for one of the reactions below. determine the value of the missing...
Questions



Biology, 17.04.2021 21:40

Social Studies, 17.04.2021 21:40

Biology, 17.04.2021 21:40


Mathematics, 17.04.2021 21:40

History, 17.04.2021 21:40





Mathematics, 17.04.2021 21:40


Mathematics, 17.04.2021 21:40


Mathematics, 17.04.2021 21:40



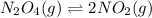
![Kc=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0108/5157/7c0a3.png)
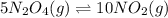
![Kc^'=\frac{[NO_2]^1^0}{[N_2O_4]^5}](/tpl/images/0108/5157/10de9.png)
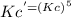
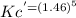
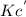 = 6.63
= 6.63

