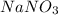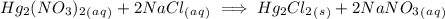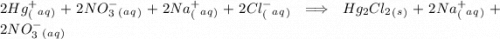Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Write complete ionic equation to show the reaction of aqueous hg2(no3)2 with aqueous sodium chloride...
Questions

Biology, 19.11.2020 19:40





Advanced Placement (AP), 19.11.2020 19:40

History, 19.11.2020 19:40


Mathematics, 19.11.2020 19:40


Mathematics, 19.11.2020 19:40


Physics, 19.11.2020 19:40

History, 19.11.2020 19:40




Mathematics, 19.11.2020 19:40


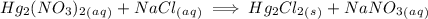 .
.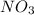 molecules on left hand side and 1 on the right hand side. So we add a coefficient of 2 on both the
molecules on left hand side and 1 on the right hand side. So we add a coefficient of 2 on both the 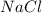 and
and