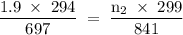Chemistry, 20.07.2019 05:30 fantasticjrod
Asample of gas (1.9 mol) is in a flask at 21 °c and 697 mm hg. the flask is opened and more gas is added to the flask. the new pressure is 841 mm hg and the temperature is now 26 °c. there are now mol of gas in the flask.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Asample of gas (1.9 mol) is in a flask at 21 °c and 697 mm hg. the flask is opened and more gas is a...
Questions

Mathematics, 04.06.2020 13:06


World Languages, 04.06.2020 13:06


Mathematics, 04.06.2020 13:06

Mathematics, 04.06.2020 13:06

Computers and Technology, 04.06.2020 13:06



Mathematics, 04.06.2020 13:06


English, 04.06.2020 13:06




Computers and Technology, 04.06.2020 13:06


Mathematics, 04.06.2020 13:06


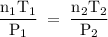
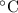 = 294 K
= 294 K