
Match each vocabulary word with its definition. match the items in the left column to the items in the right column. 1. one mole of any gas at stp will occupy 22.4 l atomic mass 2. the sum of the protons and neutrons in an atom mole 3. 6.02 x 1023; the number of atoms, molecules, or particles in a mole mass number 4. atoms of the same element that have different numbers of neutrons avogadro's hypothesis 5. the relative mass of an atom as compared to the carbon-12 atom isotopes 6. the amount of a substance that contains the same number of atoms, molecules, or particles as 12 g of carbon-12 avogadro's number 7. the mass of an element or molecule in grams per mole (g/mol) molar mass

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2


Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Match each vocabulary word with its definition. match the items in the left column to the items in t...
Questions

Chemistry, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31


Computers and Technology, 29.12.2019 23:31





Social Studies, 29.12.2019 23:31




Computers and Technology, 29.12.2019 23:31


Mathematics, 29.12.2019 23:31

English, 29.12.2019 23:31




Mathematics, 29.12.2019 23:31

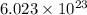 of particles.
of particles.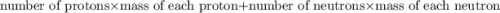 . It is a integer.
. It is a integer.

