
The nucleus of an atom is dense and positively charged. what was observed when positively charged particles were radiated onto a gold atom during rutherford's experiment because of this? negative charges were concentrated at the center of the atom. particles that struck the center of the atom were repelled. particles that struck the edges of the atom were repelled. positive charges were distributed all over the atom.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3


Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
The nucleus of an atom is dense and positively charged. what was observed when positively charged pa...
Questions

Mathematics, 03.08.2019 08:10

English, 03.08.2019 08:10

Mathematics, 03.08.2019 08:10

English, 03.08.2019 08:10


Mathematics, 03.08.2019 08:10

Mathematics, 03.08.2019 08:10



Mathematics, 03.08.2019 08:10

Social Studies, 03.08.2019 08:10

History, 03.08.2019 08:10

History, 03.08.2019 08:10


History, 03.08.2019 08:10

Biology, 03.08.2019 08:10

History, 03.08.2019 08:10


History, 03.08.2019 08:10

English, 03.08.2019 08:10

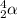 .
.

