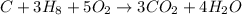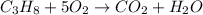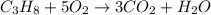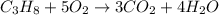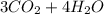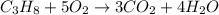Chemistry, 21.07.2019 12:00 adeliabujang8881
If the reactants on the left side of a chemical equation are c3h8 + 5o2, the products in a balanced equation could be a) 4co2 + 3h2o b) 3co2 + 4h2o c) 2co2 + 3h2o d) 3co + 4h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

You know the right answer?
If the reactants on the left side of a chemical equation are c3h8 + 5o2, the products in a balanced...
Questions


Mathematics, 29.09.2021 21:40

Biology, 29.09.2021 21:40





Spanish, 29.09.2021 21:40

Spanish, 29.09.2021 21:40


Geography, 29.09.2021 21:40


Mathematics, 29.09.2021 21:40


History, 29.09.2021 21:40



English, 29.09.2021 21:40

Biology, 29.09.2021 21:40

History, 29.09.2021 21:40