
Chemistry, 22.07.2019 00:50 bullsfan4584
Consider the chemical equilibrium of the following reaction ch3cooh ch3coo– (aq) + h+(aq) what will happen to the chemical equilibrium of the solution if ch3coona is added? the equilibrium will shifts to the right. the equilibrium will shifts to the left. the equilibrium will be unaffected. the equilibrium will be lost.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Consider the chemical equilibrium of the following reaction ch3cooh ch3coo– (aq) + h+...
Questions


Mathematics, 12.07.2019 03:00


Mathematics, 12.07.2019 03:00


Mathematics, 12.07.2019 03:00

Mathematics, 12.07.2019 03:00

Arts, 12.07.2019 03:00

Mathematics, 12.07.2019 03:00

Physics, 12.07.2019 03:00




Health, 12.07.2019 03:00



Mathematics, 12.07.2019 03:00


Biology, 12.07.2019 03:00

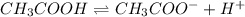
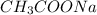 is added it will dissociate to give
is added it will dissociate to give 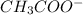 and
and 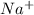
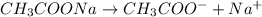
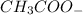 decrease i.e. the dissociation of
decrease i.e. the dissociation of 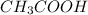 will be further depressed i.e the equilibrium will shift to the left.
will be further depressed i.e the equilibrium will shift to the left.

