
Chemistry, 22.07.2019 18:50 haileysmile2006
The specific heat of copper is 0.385 j/(g · °c). if 34.2 g of copper, initially at 24.0°c, absorbs 4.689 kj, what will be the final temperature of the copper?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
The specific heat of copper is 0.385 j/(g · °c). if 34.2 g of copper, initially at 24.0°c, absorbs 4...
Questions

Spanish, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50


Mathematics, 03.11.2020 18:50

Advanced Placement (AP), 03.11.2020 18:50

Computers and Technology, 03.11.2020 18:50


Mathematics, 03.11.2020 18:50

Business, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50


Spanish, 03.11.2020 18:50




World Languages, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50

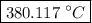
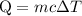 …… (1)
…… (1) is the change in temperature of copper.
is the change in temperature of copper.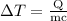 …… (2)
…… (2)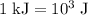
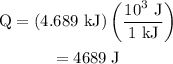
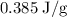 .
.
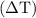 can be calculated as follows:
can be calculated as follows: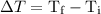 …… (3)
…… (3)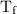 is the final temperature.
is the final temperature.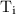 is the initial temperature.
is the initial temperature.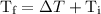 …… (4)
…… (4)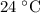 .
.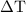 is
is 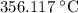
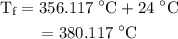
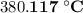 .
.


