Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
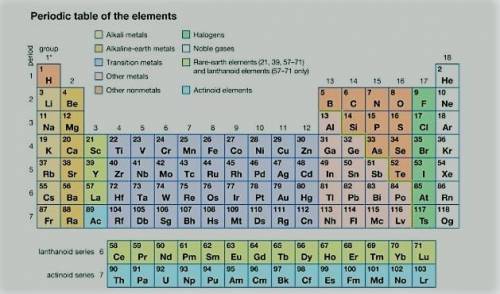
Compared to the atoms of nonmetals in period 3, the atoms of metals in period 3 have
1. fewer...
1. fewer...
Questions

Chemistry, 24.04.2020 14:15

Mathematics, 24.04.2020 14:15



Mathematics, 24.04.2020 14:15


Engineering, 24.04.2020 14:16

Mathematics, 24.04.2020 14:16



Biology, 24.04.2020 14:16




Mathematics, 24.04.2020 14:16


Mathematics, 24.04.2020 14:16

Mathematics, 24.04.2020 14:16


Mathematics, 24.04.2020 14:16