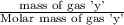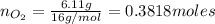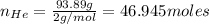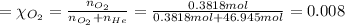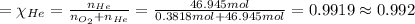Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2


Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
Agas mixture called heliox, 6.11% o2 and 93.89% he by mass, is used in scuba tanks for descents more...
Questions


Biology, 03.06.2021 17:40






Mathematics, 03.06.2021 17:40




Mathematics, 03.06.2021 17:40


Biology, 03.06.2021 17:40

Mathematics, 03.06.2021 17:40


Mathematics, 03.06.2021 17:40

Mathematics, 03.06.2021 17:40


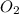 in the mixture is 0.008.
in the mixture is 0.008.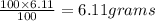
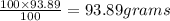
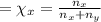
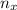 = moles of gas 'x'=
= moles of gas 'x'=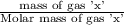
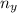 = moles of gas 'y'=
= moles of gas 'y'=