Chemistry, 23.07.2019 19:30 alexcarrasco5903
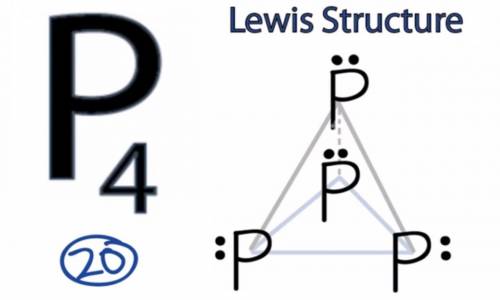
White phosphorous has the chemical formula p4(s). a p4 molecule has 20 valence electrons. draw a lewis formula for a white phosphorous molecule in which none of the atoms violates the octet rule and the formal charge on each atom is zero. there are no pi bonds in the structure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2
You know the right answer?
White phosphorous has the chemical formula p4(s). a p4 molecule has 20 valence electrons. draw a lew...
Questions



Mathematics, 23.01.2021 01:00


Social Studies, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

English, 23.01.2021 01:00

English, 23.01.2021 01:00


Mathematics, 23.01.2021 01:00



Mathematics, 23.01.2021 01:00

Biology, 23.01.2021 01:00

Geography, 23.01.2021 01:00

Chemistry, 23.01.2021 01:00

History, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00