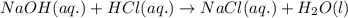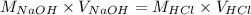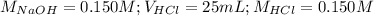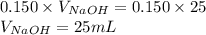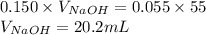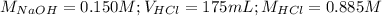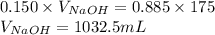Chemistry, 23.07.2019 20:10 mrylenastewart
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric acid. the neutralization reaction is: naoh(aq) + hcl(aq) → h2o(l) + nacl(aq) 25 ml of a 0.150 m hcl solution 55 ml of a 0.055 m hcl solution 175 ml of a 0.885 m hcl solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric aci...
Questions


Physics, 18.03.2021 02:30





Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

English, 18.03.2021 02:30

Arts, 18.03.2021 02:30




Social Studies, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30


Arts, 18.03.2021 02:30

Computers and Technology, 18.03.2021 02:30