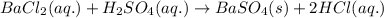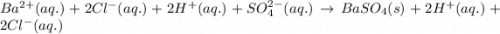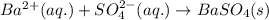Chemistry, 24.07.2019 02:20 hannah5143
Write a balanced net ionic equation for the following reaction: bacl2(aq) + h2so4 (aq) -- baso4(s) + hcl (aq) show your work

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

You know the right answer?
Write a balanced net ionic equation for the following reaction: bacl2(aq) + h2so4 (aq) -- baso4(s)...
Questions

Mathematics, 04.12.2019 06:31



History, 04.12.2019 06:31



Physics, 04.12.2019 06:31

Mathematics, 04.12.2019 06:31

History, 04.12.2019 06:31

Mathematics, 04.12.2019 06:31




History, 04.12.2019 06:31

English, 04.12.2019 06:31

Mathematics, 04.12.2019 06:31

History, 04.12.2019 06:31

Mathematics, 04.12.2019 06:31