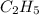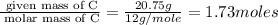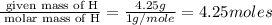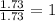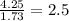Chemistry, 24.07.2019 09:00 whiteshawn02
Asample of a hydrocarbon contains 20.75 g c and 4.25 g h. its molar mass is 58.04 g/mol. what is its empirical formula c2h5 ch2 ch c5h

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 14:30
When carbon disulfide is formed from its elements, heat is absorbed. calculate the amount of heat in (kj) absorbed when 5.66g of carbon disulfide is formed
Answers: 3
You know the right answer?
Asample of a hydrocarbon contains 20.75 g c and 4.25 g h. its molar mass is 58.04 g/mol. what is its...
Questions

Biology, 11.06.2021 01:40

Health, 11.06.2021 01:40

History, 11.06.2021 01:40

Advanced Placement (AP), 11.06.2021 01:40

English, 11.06.2021 01:40



Health, 11.06.2021 01:40

Mathematics, 11.06.2021 01:40



Mathematics, 11.06.2021 01:40

Physics, 11.06.2021 01:40

English, 11.06.2021 01:50

Mathematics, 11.06.2021 01:50

Mathematics, 11.06.2021 01:50

Mathematics, 11.06.2021 01:50



Business, 11.06.2021 01:50