
Chemistry, 24.07.2019 10:30 graymonky12
Balance the following equation. then determine the ratio for the products kcl and o2 generated during the decomposition of potassium chlorate. kclo3 kcl + o2 1: 1 2: 2 4: 3 2: 3 3: 2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Balance the following equation. then determine the ratio for the products kcl and o2 generated durin...
Questions



Mathematics, 03.04.2020 02:44

Mathematics, 03.04.2020 02:44

Mathematics, 03.04.2020 02:44

Advanced Placement (AP), 03.04.2020 02:44






Engineering, 03.04.2020 02:44


Mathematics, 03.04.2020 02:44

Mathematics, 03.04.2020 02:44

Mathematics, 03.04.2020 02:44



Mathematics, 03.04.2020 02:44

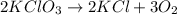
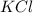 and 3 moles of
and 3 moles of 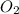 .
.

