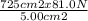Chemistry, 07.11.2019 16:31 welathyasf
Hi!me with 9 science 90 points and !
1. what causes the weight of an object to change depending on if it is on earth or the moon?
the change in mass
the distance from the sun
the distance from earth
the acceleration due to gravity
2. the mass of a mouse is around 22 grams (0.022 kg). what is the weight of a mouse at sea level on earth?
22 g
0.022 kg
22 n
0.22 n
3. the mass of a mouse is around 22 grams (0.022 kg). what is the weight of a mouse on the moon?
0.022 n
22 g
0.035 n
0.022 kg
4. the bottom of a container has an area of 25 cm2. if 6.5 newtons of water are poured into the container, what is the pressure exerted on the bottom?
0.26 n/cm^2
3.8 n/cm^2
2.6 n/cm^2
162.5 n/cm^2
5. as a fish swims deeper into the ocean, the pressure on its body . (increases, decreases)
6. use the formula for pressure to determine the weight of a box that creates a pressure of 565 n/cm^2 when resting on a 15.0 cm^2 surface.
weight = 3.84 n
weight = 377 n
weight = 37.7 n
weight = 8,475 n
7. when pressure is applied to a liquid, it will change its but not its
shape, volume
volume, shape
shape, temperature
temperature, shape
8. select all that apply. (select all that are correct, probably more then one)
hydraulic systems work because
liquids are incompressible
pressure is transmitted equally throughout a liquid
gases are incompressible
pressure and force are the same thing
9. a hydraulic system is designed to lift cars for inspection in a service station. the narrow end of the system has a surface area of 5.00 cm^2, and the lift platform (the wide end) has a surface area of 725 cm^2. if a force of 81.0 newtons is applied to the narrow end, how much upward lift force will be exerted at the wide end?
use the following formula:
(picture below)
0.559 n
11,745 n
44.75 n
293,625 n

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
Hi!me with 9 science 90 points and !
1. what causes the weight of an object to change...
1. what causes the weight of an object to change...
Questions



Social Studies, 12.10.2020 22:01




Mathematics, 12.10.2020 22:01

Computers and Technology, 12.10.2020 22:01

English, 12.10.2020 22:01



History, 12.10.2020 22:01



Mathematics, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01

History, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01


Mathematics, 12.10.2020 22:01