
Chemistry, 25.07.2019 02:00 JocelynC7237
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentration of oh− ions.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentrati...
Questions


English, 25.02.2021 07:40

Biology, 25.02.2021 07:40


Mathematics, 25.02.2021 07:40


Mathematics, 25.02.2021 07:40

English, 25.02.2021 07:40

English, 25.02.2021 07:40

Biology, 25.02.2021 07:40

Mathematics, 25.02.2021 07:40

Social Studies, 25.02.2021 07:40

Mathematics, 25.02.2021 07:40





English, 25.02.2021 07:40


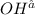 is 0.8 M.
is 0.8 M.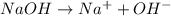
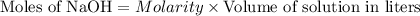
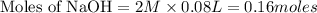
![[OH^-]](/tpl/images/0129/3993/b2910.png)


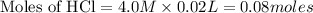
![[H^+]](/tpl/images/0129/3993/07acb.png)

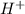 will react with 0.08 moles of
will react with 0.08 moles of 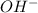 and (0.16-0.08)= 0.08 moles of
and (0.16-0.08)= 0.08 moles of ![[OH^]-=\frac{moles}{\text {Volume in L}}=\frac{0.08}{0.1}=0.8M](/tpl/images/0129/3993/83da4.png)


