
Chemistry, 27.10.2019 16:43 lashaaungas
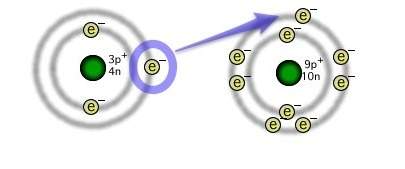
The illustration depicts the formation of an ionic chemical bond between lithium and fluorine atoms. why is the resulting compound more stable than the individual atoms?
a) the shared electron from lithium to fluorine provides each atom with a full outer energy level.
b) the shared electron from lithium to fluorine provides each atom with an empty outer energy level.
c) the transferred electron from lithium to fluorine provides each atom with a full outer energy level.
d) the transferred electron from lithium to fluorine provides each atom with an empty outer energy level.
< 3

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2
You know the right answer?
The illustration depicts the formation of an ionic chemical bond between lithium and fluorine atoms....
Questions








Computers and Technology, 22.01.2020 20:31






Mathematics, 22.01.2020 20:31


Computers and Technology, 22.01.2020 20:31






