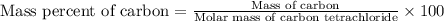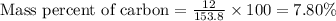Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Calculate the mass percent of carbon (c) in carbon tetrachloride, (ccl4), if the molar mass of carbo...
Questions


Mathematics, 19.02.2021 06:20




Mathematics, 19.02.2021 06:20

Chemistry, 19.02.2021 06:20


World Languages, 19.02.2021 06:20

Mathematics, 19.02.2021 06:20


English, 19.02.2021 06:20

Mathematics, 19.02.2021 06:20


English, 19.02.2021 06:20


Geography, 19.02.2021 06:20



 , there are 1 carbon atoms and 4 chlorine atoms.
, there are 1 carbon atoms and 4 chlorine atoms.