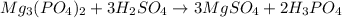Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Magnesium phosphate reacts with sulfuric acid to form magnesium sulfate and phosphoric acid. what is...
Questions

Mathematics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

History, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Biology, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Physics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

English, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01


Mathematics, 10.09.2020 03:01



Spanish, 10.09.2020 03:01

French, 10.09.2020 03:01

Computers and Technology, 10.09.2020 03:01