
Chemistry, 25.07.2019 22:40 marlandwilliams10
Consider the balanced chemical equation, mgcl2(aq) + 2agf(aq) → 2agcl(s) + mgf2(aq) if both reactants are completely consumed and we produce 20 formula units of agcl, how many ions of mg and f did we start with?

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
Consider the balanced chemical equation, mgcl2(aq) + 2agf(aq) → 2agcl(s) + mgf2(aq) if both reactant...
Questions


History, 29.01.2021 07:00

Spanish, 29.01.2021 07:00





Biology, 29.01.2021 07:00

Mathematics, 29.01.2021 07:00

Social Studies, 29.01.2021 07:00

Mathematics, 29.01.2021 07:00





Spanish, 29.01.2021 07:00

Mathematics, 29.01.2021 07:00


Mathematics, 29.01.2021 07:00

English, 29.01.2021 07:00

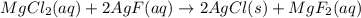
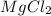 .
.

