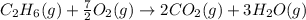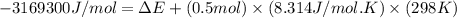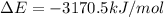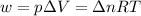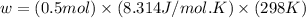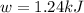Chemistry, 26.07.2019 04:00 tonytashaqua
Calculate the work (w) and δeo, in kj, at 298 k and 1 atm pressure, for the combustion of one mole of c6h6 (g). first write and balance the equation. the products will be co2 (g) and h2o (g). the value of δho for this reaction is -3169.3 kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Calculate the work (w) and δeo, in kj, at 298 k and 1 atm pressure, for the combustion of one mole o...
Questions


Mathematics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

Arts, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00


History, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00





Computers and Technology, 25.01.2021 01:00



Mathematics, 25.01.2021 01:00

Health, 25.01.2021 01:00

English, 25.01.2021 01:00

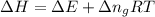
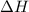 = change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol
= change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol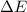 = change in internal energy
= change in internal energy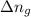 = change in moles
= change in moles