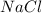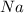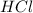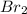Chemistry, 26.07.2019 09:00 jordandabrat
The bonds in bromine, br2, allows for complete dissolving but not dissociation cyclohexane. bromine will not dissolve or dissociate in water. a) ionic b) metallic c) polar covalent d) nonpolar covalent

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
The bonds in bromine, br2, allows for complete dissolving but not dissociation cyclohexane. bromine...
Questions

History, 31.07.2019 11:30


Biology, 31.07.2019 11:30



Mathematics, 31.07.2019 11:30



Biology, 31.07.2019 11:30

Mathematics, 31.07.2019 11:30






History, 31.07.2019 11:30



Social Studies, 31.07.2019 11:30