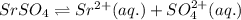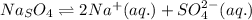Chemistry, 26.07.2019 16:20 brandydailey24pe8r24
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfate is added because? 1)the addition of sulfate ions shifts equilibrium to the left. 2)the addition of sodium ions shifts equilibrium to the left. 3)the addition of sulfate ions shifts equilibrium to the right. 4)the addition of sodium ions shifts equilibrium to the right.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfate is added because?...
Questions

English, 23.05.2020 05:00

History, 23.05.2020 05:00



History, 23.05.2020 05:00








English, 23.05.2020 05:00

Mathematics, 23.05.2020 05:00

Mathematics, 23.05.2020 05:00

Biology, 23.05.2020 05:00

History, 23.05.2020 05:00


English, 23.05.2020 05:00

Geography, 23.05.2020 05:00