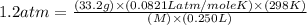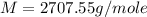Chemistry, 27.07.2019 18:40 krazziekidd2p845ri
An aqueous solution of a soluble compound (a nonelectrolyte) is prepared by dissolving 33.2 g of the compound in sufficient water to form 250 ml of solution. the solution has an osmotic pressure of 1.2 atm at 25 °c. what is the molar mass (g/mole) of the compound?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
An aqueous solution of a soluble compound (a nonelectrolyte) is prepared by dissolving 33.2 g of the...
Questions




Mathematics, 04.06.2020 13:21











Mathematics, 04.06.2020 13:21

Mathematics, 04.06.2020 13:21

Health, 04.06.2020 13:21


Mathematics, 04.06.2020 13:21

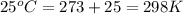
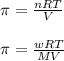
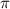 = osmotic pressure
= osmotic pressure