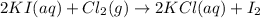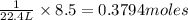Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Assume that 8.5 l of iodine gas (i2) are produced at stp according to the following balanced equatio...
Questions

Mathematics, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50

Computers and Technology, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50

Law, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50



Mathematics, 08.01.2021 21:50

Physics, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50

English, 08.01.2021 21:50

Spanish, 08.01.2021 21:50



Mathematics, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50

Mathematics, 08.01.2021 21:50