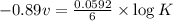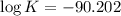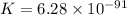Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

You know the right answer?
What is the value of the equilibrium constant for this redox reaction? 2cr3+(aq) + 3sn2+ (aq) 2cr(s...
Questions


Mathematics, 20.12.2019 17:31


Mathematics, 20.12.2019 17:31


English, 20.12.2019 17:31


History, 20.12.2019 17:31

Mathematics, 20.12.2019 17:31

Biology, 20.12.2019 17:31

Mathematics, 20.12.2019 17:31


English, 20.12.2019 17:31


Mathematics, 20.12.2019 17:31


Physics, 20.12.2019 17:31


Mathematics, 20.12.2019 17:31

Spanish, 20.12.2019 17:31

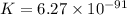
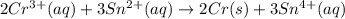
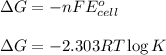
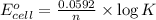 ........(1)
........(1)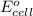 = cell potential = -0.89 v
= cell potential = -0.89 v