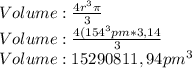Chemistry, 01.08.2019 05:00 Tyrant4life
Given the atomic radius of neon, 0.69 å, and knowing that a sphere has a volume of 4πr3/3, calculate the fraction of space that ne atoms occupy in a sample of neon at stp.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 23.06.2019 08:40
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3

Chemistry, 23.06.2019 11:20
Sandy is building a small toy car. he wants to use a balloon to power the toy car. he fills a balloon with air and then attaches a straw to the balloon. he tapes the balloon-straw combination to the car and then releases the end of the balloon. the toy moves forward as the air from the balloon comes out the back of the straw. what can sandy do to make the toy car move faster? a) use less air in the balloon. b) blow up the balloon more. c) use a longer straw. d) use larger tires.
Answers: 2
You know the right answer?
Given the atomic radius of neon, 0.69 å, and knowing that a sphere has a volume of 4πr3/3, calculate...
Questions

Social Studies, 16.12.2020 04:30


Spanish, 16.12.2020 04:30

History, 16.12.2020 04:30

English, 16.12.2020 04:30




Mathematics, 16.12.2020 04:30

Chemistry, 16.12.2020 04:30


Mathematics, 16.12.2020 04:30






History, 16.12.2020 04:30


Mathematics, 16.12.2020 04:30