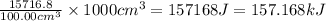Chemistry, 01.08.2019 08:20 tintlemax6256
Astudent added 5.350 g of ammonium chloride to 100.00 cm3 of water. the initial temperature of the water was 25.55℃ but it decreased to 21.79℃. calculate the enthalpy change that would occur when 1 mol of the solute is added to 1.0000 dm3 of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Astudent added 5.350 g of ammonium chloride to 100.00 cm3 of water. the initial temperature of the w...
Questions


Computers and Technology, 18.06.2021 02:10

Biology, 18.06.2021 02:10







Mathematics, 18.06.2021 02:10




Mathematics, 18.06.2021 02:10


Mathematics, 18.06.2021 02:10


Mathematics, 18.06.2021 02:10


Mathematics, 18.06.2021 02:10

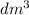 of water is 157.168 kJ/mol.
of water is 157.168 kJ/mol.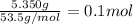
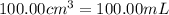
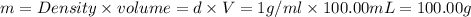
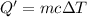
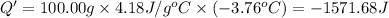
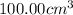 of water .
of water .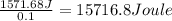
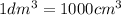
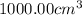 of water :
of water :