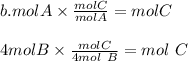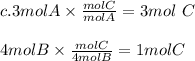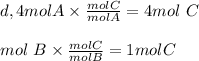Chemistry, 01.08.2019 21:40 milkshakegrande101
Look at the following hypothetical reaction: a + 4b - c + 3d. assume that the equation is balanced. which of the following mole ratios will produce the most product? a) a/b b) a/4b c) 3a/4b d) 4a/b

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
Look at the following hypothetical reaction: a + 4b - c + 3d. assume that the equation is balanced....
Questions

Biology, 25.01.2021 22:20


Mathematics, 25.01.2021 22:20

Physics, 25.01.2021 22:20



Arts, 25.01.2021 22:20

Chemistry, 25.01.2021 22:20


Arts, 25.01.2021 22:20


Mathematics, 25.01.2021 22:20


Computers and Technology, 25.01.2021 22:20


Computers and Technology, 25.01.2021 22:20




 .
.