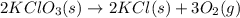Chemistry, 28.09.2019 14:10 mckadams02
Potassium chlorate (kclo3) decomposes in a reaction described by this chemical equation: 2kclo3(s) → 2kcl(s) + 3o2(g) if 36.0 grams of potassium chlorate enter into this reaction, what is the total mass of the two products? 90.0 grams 54.0 grams 18.0 grams 36.0 grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
Potassium chlorate (kclo3) decomposes in a reaction described by this chemical equation: 2kclo3(s)...
Questions


Mathematics, 13.02.2021 03:50


Social Studies, 13.02.2021 03:50

Mathematics, 13.02.2021 03:50

English, 13.02.2021 03:50



Mathematics, 13.02.2021 03:50

Mathematics, 13.02.2021 03:50

Mathematics, 13.02.2021 03:50

Chemistry, 13.02.2021 03:50


Social Studies, 13.02.2021 03:50


Mathematics, 13.02.2021 03:50

Mathematics, 13.02.2021 03:50


Mathematics, 13.02.2021 03:50

Geography, 13.02.2021 03:50