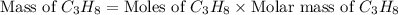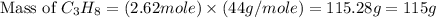Chemistry, 02.08.2019 08:10 FailingstudentXD
What mass of propane (c3h8) is needed to produce 346 g carbon dioxide in the following reaction? a. 346 g c3h8 b. 1.86 g c3h8 c. 5075 g c3h8 d. 115 g c3h8

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
What mass of propane (c3h8) is needed to produce 346 g carbon dioxide in the following reaction? a....
Questions


Mathematics, 15.04.2021 05:40

Mathematics, 15.04.2021 05:40


History, 15.04.2021 05:40


Spanish, 15.04.2021 05:40





Mathematics, 15.04.2021 05:40



Mathematics, 15.04.2021 05:40

Mathematics, 15.04.2021 05:40

History, 15.04.2021 05:40

Social Studies, 15.04.2021 05:40


Mathematics, 15.04.2021 05:40

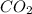 = 346 g
= 346 g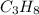 = 44 g/mole
= 44 g/mole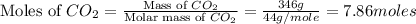
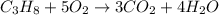
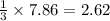 moles of
moles of