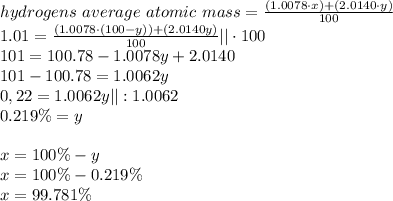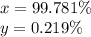Chemistry, 21.08.2019 18:00 kristadwsn
Hydrogen is found primarily as two isotopes in nature: (1.0078u) and (2.0140u). caluclate the percentage abundance of each isotope based on hydrogens average atomic mass

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
Hydrogen is found primarily as two isotopes in nature: (1.0078u) and (2.0140u). caluclate the perce...
Questions

Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00



History, 20.01.2021 01:00

Biology, 20.01.2021 01:00


Mathematics, 20.01.2021 01:00


English, 20.01.2021 01:00


English, 20.01.2021 01:00


Mathematics, 20.01.2021 01:00

English, 20.01.2021 01:00

Chemistry, 20.01.2021 01:00


Social Studies, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00