
Chemistry, 04.08.2019 16:00 NeonPlaySword
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm o2=32 g/mol; mm fe2o3=159.70 g/mol) if 63.98 g of oxygen gas is completely consumed, how many moles of iron (iii) oxide are formed? a. 1.333 mol b. 3071 mol c. 2.999 mol d. 6812 mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1


Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm...
Questions


Mathematics, 03.03.2021 16:50

Mathematics, 03.03.2021 16:50

Mathematics, 03.03.2021 16:50

Biology, 03.03.2021 16:50




Social Studies, 03.03.2021 16:50

Spanish, 03.03.2021 16:50




Mathematics, 03.03.2021 16:50

Mathematics, 03.03.2021 16:50





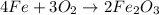
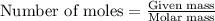
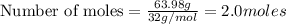
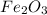 .
.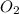 produces 2 moles of
produces 2 moles of 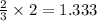 moles of
moles of 

