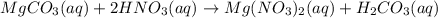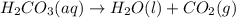Chemistry, 04.08.2019 16:30 aharvitt0417
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid form. carbonic acid then breaks down into water and carbon dioxide. what two types of reactions take place in this process?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

You know the right answer?
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid...
Questions






Mathematics, 05.06.2020 07:01

History, 05.06.2020 07:01

Mathematics, 05.06.2020 07:01

English, 05.06.2020 07:01



Mathematics, 05.06.2020 07:01

Mathematics, 05.06.2020 07:01


Biology, 05.06.2020 07:01

Mathematics, 05.06.2020 07:01

Mathematics, 05.06.2020 07:01

Mathematics, 05.06.2020 07:01


Chemistry, 05.06.2020 07:01