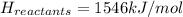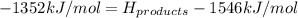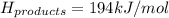Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants is 1546 kj/mol. calculate the total bonding energy of the products and decide whether the reaction is endothermic or exothermic a)84 kj/mol, endothermic b)194 kj/mol, endothermic c) 84 kj/mol, exothermic d)194 kj/mol, exothermic

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants...
Questions


Mathematics, 27.04.2020 03:14

Physics, 27.04.2020 03:14

Mathematics, 27.04.2020 03:14

Mathematics, 27.04.2020 03:14

English, 27.04.2020 03:14

English, 27.04.2020 03:14

Mathematics, 27.04.2020 03:14


Mathematics, 27.04.2020 03:15

Mathematics, 27.04.2020 03:15





Mathematics, 27.04.2020 03:15


Mathematics, 27.04.2020 03:15


Mathematics, 27.04.2020 03:15

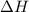 for the reaction comes out to be negative.
for the reaction comes out to be negative.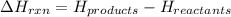
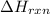 = 1352kJ/mol[/tex]
= 1352kJ/mol[/tex]