
Chemistry, 04.08.2019 22:30 kyliemorgan8623
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg ab (fast) and the rate = k[a][b] step 2: ab + b mc016-2.jpg ab2 (slow) and the rate = k[ab][b] step 3: ab2 + b mc016-3.jpg ab3 (fast) and the rate = k[ab2][b] overall: a + 3b mc016-4.jpg ab3 and the rate = k[a][b]2 which explains why the rate law for the overall equation is not the same as the rate equation for the rate-determining step?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg...
Questions





History, 05.11.2020 20:00

Physics, 05.11.2020 20:00

Mathematics, 05.11.2020 20:00



Mathematics, 05.11.2020 20:00


Mathematics, 05.11.2020 20:00

Mathematics, 05.11.2020 20:00

Chemistry, 05.11.2020 20:00

Mathematics, 05.11.2020 20:00

History, 05.11.2020 20:00

Geography, 05.11.2020 20:00

English, 05.11.2020 20:00

Mathematics, 05.11.2020 20:00

Chemistry, 05.11.2020 20:10

![A+B\rightleftharpoons AB(fast)\\Rate=K[A][B]](/tpl/images/0171/0396/8b588.png)
![AB+B\rightarrow AB_2(slow)\\Rate=K[AB][B]](/tpl/images/0171/0396/fb2ef.png)
![AB_2+B\rightleftharpoons AB_3(fast)\\Rate=K[AB_2][B]](/tpl/images/0171/0396/ac6cb.png)
![Rate=K[A][B]^2](/tpl/images/0171/0396/92d0b.png)
![Rate=K[AB][B]](/tpl/images/0171/0396/058ce.png) ...........(A)
...........(A)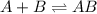
![K=\frac{[AB]}{[A][B]}](/tpl/images/0171/0396/6ec78.png)
![[AB]=K[A][B]](/tpl/images/0171/0396/ad806.png)
![Rate=KK[A][B][B]](/tpl/images/0171/0396/3919b.png)
![Rate=K'[A][B]^2](/tpl/images/0171/0396/8db87.png)



