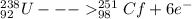proton produces nuclide x and a neutron. what is nuclide x?

Chemistry, 25.08.2019 01:30 lathwkuster
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
answers:
magnesium-24
magnesium-23
neon-23
s
odium-24
none of the above
the isotope p
has a half-life of 14.3 days. if a sample originally contained 1.00 g of p,
how much was left after 43 days?
answers:
0.250 g
0.125 g
0.750 g
0.500 g
identify x in the reaction
below.
u
+ c
→ cf
+ x
answers:
1
alpha particle
3
protons
6
neutrons
6
electrons
a .20 gram sample of c-14 was allowed to decay for 3 half-lives. what mass
of the sample will remain? carbon-14 has a half life of 5730
years.
answers:
0.025
0.05
0.1
0
.01
0.05
the isotope cu
has a half-life of 30 s. if a sample originally contained 48 mg of cu,
how much time passed before the amount fell to 3 mg?
answers:
120 s
240s
30 s
60 s(when i did my calculation for the question above, i got 60 seconds)
what radionuclide decays to fe-56
by beta emission?
answers:
fe
co
mn
co
mnhow do i know wat it becomes. i put 57 co 27 and it's wrong.
the cf
to cf
conversion is accompanied by
answers:
an alpha
emission
a
neutron capture
an electron
capture
an electron releasei would greatly appreciate your . you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
proton produces nuclide x and a neutron. what is nuclide x?
Questions








Chemistry, 05.10.2019 02:00

History, 05.10.2019 02:00

English, 05.10.2019 02:00



English, 05.10.2019 02:00


Mathematics, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00

Social Studies, 05.10.2019 02:00

Health, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00