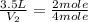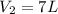The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(g). if a balloon was filled with 3.5l of ammonia gas and all of the gas decomposed, what would be the total volume of gas in the container? assume the pressure and temp remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2



Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(...
Questions

Mathematics, 10.05.2021 05:30




Chemistry, 10.05.2021 05:30


Chemistry, 10.05.2021 05:30

History, 10.05.2021 05:30

Mathematics, 10.05.2021 05:30


Mathematics, 10.05.2021 05:30

Mathematics, 10.05.2021 05:30

Mathematics, 10.05.2021 05:30

Biology, 10.05.2021 05:30

Mathematics, 10.05.2021 05:30



Mathematics, 10.05.2021 05:30


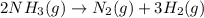
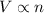
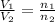
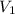 = initial volume of ammonia gas = 3.5 L
= initial volume of ammonia gas = 3.5 L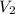 = total volume of gas in the container = ?
= total volume of gas in the container = ?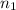 = initial moles of ammonia gas = 2 mole
= initial moles of ammonia gas = 2 mole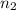 = final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles
= final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles