
Chemistry, 07.10.2019 14:30 gyexisromero10
The following structures will have a double, triple, resonating, or coordinate covalent bond. draw lewis dot structures for each and state if it is a polar or nonpolar molecule.
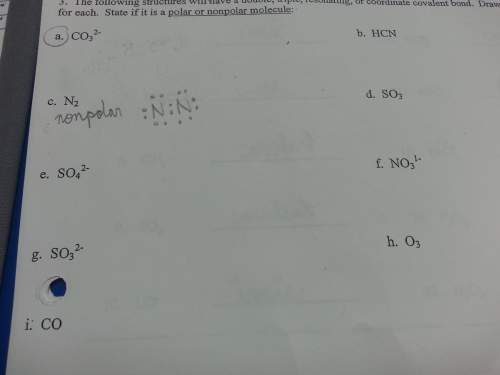

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
The following structures will have a double, triple, resonating, or coordinate covalent bond. draw l...
Questions

History, 12.10.2019 00:00

Social Studies, 12.10.2019 00:00


Biology, 12.10.2019 00:00

Arts, 12.10.2019 00:00








Mathematics, 12.10.2019 00:10

Mathematics, 12.10.2019 00:10



Mathematics, 12.10.2019 00:10


Chemistry, 12.10.2019 00:10

History, 12.10.2019 00:10



