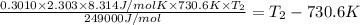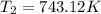Chemistry, 04.08.2019 02:00 natalieoppelt
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 hcl the activation energy is 249 kj/mol and the frequency factor is 1.6 × 1014 s−1. find the temperature at which the rate of the reaction would be twice as fast as when the reaction runs at 730.6 k. enter your answer numerically and in terms of kelvin.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

You know the right answer?
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 hcl the activation energy...
Questions

Mathematics, 24.08.2019 23:50



History, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50

Chemistry, 24.08.2019 23:50




Mathematics, 24.08.2019 23:50



Biology, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50


History, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50

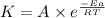
![\log \frac{K_2}{K_1}=\frac{E_a}{2.303R}\times [\frac{T_2-T_1}{T_1T_2}]](/tpl/images/0167/7789/e0c66.png)
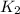 = rate of reaction at
= rate of reaction at 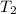
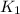 = rate of reaction at
= rate of reaction at 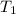
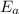 = activation energy
= activation energy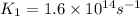
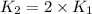
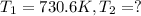
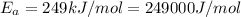
![\log \frac{2K_1}{K_1}=\frac{249000 kJ/mol}{2.303\times 8.314 J/mol K}\times [\frac{T_2-730.6 K}{730.6 K\times T_2}]](/tpl/images/0167/7789/638fd.png)
![0.3010=\frac{249000 J/mol}{2.303\times 8.314 J/mol K}\times [\frac{T_2-730.6 K}{730.6 K\times T_2}]](/tpl/images/0167/7789/3fa9a.png)