
Chemistry, 04.08.2019 00:30 bullockarwen
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) → 2mgo(s) how many moles of o2 are consumed when 0.550 mol of magnesium burns?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) → 2mgo...
Questions



Mathematics, 18.04.2020 03:02


History, 18.04.2020 03:02



Geography, 18.04.2020 03:02


Mathematics, 18.04.2020 03:02




Mathematics, 18.04.2020 03:02

Mathematics, 18.04.2020 03:02


Mathematics, 18.04.2020 03:02

Mathematics, 18.04.2020 03:02

Mathematics, 18.04.2020 03:02

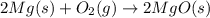
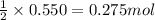 of oxygen gas.
of oxygen gas.

