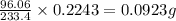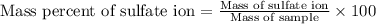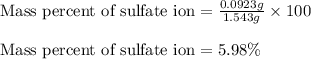Chemistry, 03.08.2019 22:30 sindy35111
A1.543 gram sample containing sulfate ion was treated with barium chloride reagent, and 0.2243 grams of barium sulfate was isolated. calculate the percentage of sulfate ion in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
A1.543 gram sample containing sulfate ion was treated with barium chloride reagent, and 0.2243 grams...
Questions

English, 14.12.2020 23:30

Health, 14.12.2020 23:30



Mathematics, 14.12.2020 23:30


Mathematics, 14.12.2020 23:30


Mathematics, 14.12.2020 23:30

Mathematics, 14.12.2020 23:30

Chemistry, 14.12.2020 23:30

Social Studies, 14.12.2020 23:30

Mathematics, 14.12.2020 23:30

Mathematics, 14.12.2020 23:30

Social Studies, 14.12.2020 23:30


Physics, 14.12.2020 23:30

Computers and Technology, 14.12.2020 23:30


Mathematics, 14.12.2020 23:30