

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3


Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
When solid borax is dissolved in water do you expect entropy of the system to increase or decrease?...
Questions




History, 21.01.2021 20:30



Business, 21.01.2021 20:30


Mathematics, 21.01.2021 20:30

Mathematics, 21.01.2021 20:30

Mathematics, 21.01.2021 20:30

Mathematics, 21.01.2021 20:30


Mathematics, 21.01.2021 20:30


Mathematics, 21.01.2021 20:30

Chemistry, 21.01.2021 20:30

History, 21.01.2021 20:30


Biology, 21.01.2021 20:30

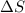 is positive.
is positive.

