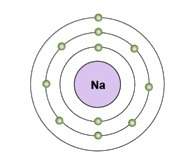
The diagram shows an electron shell model of a sodium atom.
how would the model change a...

The diagram shows an electron shell model of a sodium atom.
how would the model change as the atom forms bonds?
a. the third shell would have eight electrons after the atom gains seven electrons to fill the outermost shell.
b. the third shell would be empty so that the eight electrons in the second level would be outermost after the atom loses one electron.
c. the first and third shells would be empty so that the atom would have eight electrons in its remaining shell after the atom loses three electrons.
d. the first shell would have two electrons and the second shell would have six electrons after the atom loses three electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Questions

Computers and Technology, 16.07.2020 01:01

History, 16.07.2020 01:01







History, 16.07.2020 01:01









English, 16.07.2020 01:01




