
Chemistry, 03.08.2019 05:30 krystlemiller4307
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 . if the molar mass of the compound is 129.1 g/mol, what is the chemical formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2


Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 ....
Questions


Mathematics, 10.12.2019 12:31

History, 10.12.2019 12:31

Mathematics, 10.12.2019 12:31




Biology, 10.12.2019 12:31

Mathematics, 10.12.2019 12:31


Mathematics, 10.12.2019 12:31



Mathematics, 10.12.2019 12:31


Mathematics, 10.12.2019 12:31

Biology, 10.12.2019 12:31

Mathematics, 10.12.2019 12:31


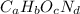 . The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.
. The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.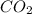 , 1.09 g of
, 1.09 g of 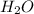 and 1.70 g of
and 1.70 g of 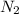 . First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.
. First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.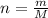
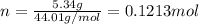
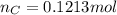
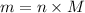

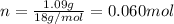


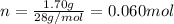
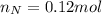

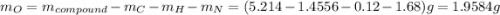
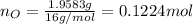
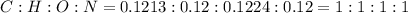

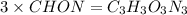
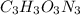 .
.


