
Chemistry, 10.10.2019 15:50 ShugarLove4363
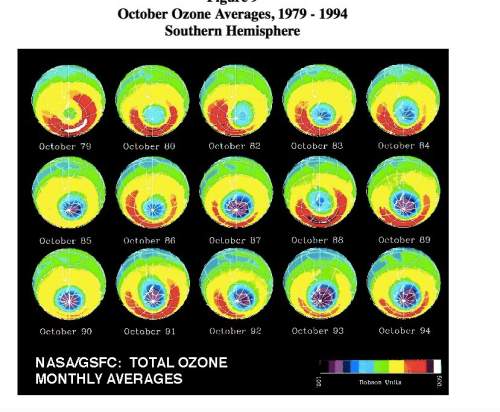
On average, one chlorine atom can destroy 100,000 ozone molecules. such destructive power is tremendous when it is related to the millions of tons of cfcs that had been produced up until that time. when rowland and molina calculated the likely effect on the ozone layer, they could not believe their figures. they consulted another scientist hal johnson who confirmed their results. in 1974, rowland and molina published their predictions about ozone depletion in the scientific journal nature. although the united states was quick to ban the production of cfcs, many other countries including britain, china, france and japan did not. look at the ozone depletion images from 1979-1994. these images show the amounts of ozone in the atmosphere above antarctica in october. the colors indicate the relative amounts of ozone present. the “red” color indicates the highest amounts and the “purple” indicates the smallest amounts. in 1994, the “ozone hole” showed to have a loss of 65% of its original ozone amounts. using the images collected on the site, describe the trends you see over time in the strength of the ozone coverage.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

Chemistry, 23.06.2019 02:50
For questions 1 and 2, consider the following experimental data.hydrogen emission lines were detected at the following wavelengths (in nm): 121.6102.697.395.093.8question 1use the electromagnetic radiation classifications below and figure 1-1 in the introductory information for this lab (in the lab manual) to determine the nf value for the experimental data provided? wavelength, ? (nm) 650 700 550 600 400 450 500 visible spectrum wavelength, ? (m) 11 10 3 10 10 10 8 10 5 10 10 -10 10 9 10 10 10 10 -12 10 microwave radio infrared x-ray ultraviolet gamma 1020 1019 1018 1 1016 015 1014 01 12 109108 frequency, v (hz)a.1b. 2c. 3d. 4e. 5question 2using the data for the emission line with the longest wavelength, the known value of nf (from question 1 in this prelab), and the value of ni (deduced from the ? and nf values) calculate the rydberg constant for hydrogen (rh) in units of m-1.a) 1.097 x 10-11 m-1b) 5.921 x 107 m-1c) 1.097 x 10-2 m-1d) 9.252 x 106 m-1e) 1.097 x 107 m-1
Answers: 3
You know the right answer?
On average, one chlorine atom can destroy 100,000 ozone molecules. such destructive power is tremend...
Questions


Mathematics, 10.09.2019 04:10


English, 10.09.2019 04:10



Spanish, 10.09.2019 04:10









Computers and Technology, 10.09.2019 04:10






