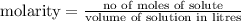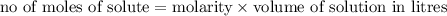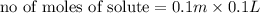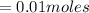Chemistry, 03.08.2019 01:30 alyssawage
Calculate the number of moles of kool aid powder needed to make 100ml of a 0.1 m solution? 0.1m=x mol c12h22o11/0.1l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
Calculate the number of moles of kool aid powder needed to make 100ml of a 0.1 m solution? 0.1m=x m...
Questions

Mathematics, 28.07.2019 02:00


World Languages, 28.07.2019 02:00

Mathematics, 28.07.2019 02:00

English, 28.07.2019 02:00



Biology, 28.07.2019 02:00



English, 28.07.2019 02:00

Mathematics, 28.07.2019 02:00

Mathematics, 28.07.2019 02:00


Biology, 28.07.2019 02:00


Chemistry, 28.07.2019 02:00

Chemistry, 28.07.2019 02:00

History, 28.07.2019 02:00