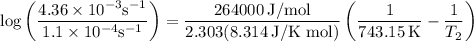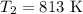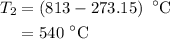Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

You know the right answer?
The rate constant of a reaction is 1.1 × 10-4 s-1 at 470 °c, and the activation energy is 264 kj/mol...
Questions


Chemistry, 24.01.2021 14:00


Advanced Placement (AP), 24.01.2021 14:00


Physics, 24.01.2021 14:00


Social Studies, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00

History, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00




History, 24.01.2021 14:00

History, 24.01.2021 14:00

English, 24.01.2021 14:00

English, 24.01.2021 14:00

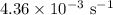 comes out to be
comes out to be  .
.
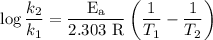 …… (1)
…… (1)
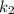 is rate constant at temperature
is rate constant at temperature 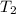 .
.
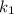 is rate constant temperature
is rate constant temperature 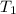 .
.
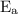 is activation energy.
is activation energy.
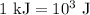
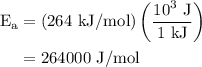
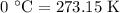
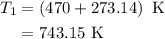
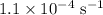 .
.